Chemistry
Exercise 9
9. Understand the fundamental concepts in organic chemistry.
A basic knowledge of organic chemistry is a minimum requirement for understanding environmental chemistry. Nearly all the synthetic materials of the 21st century are organic in nature and are closely linked to the petrochemical industry.
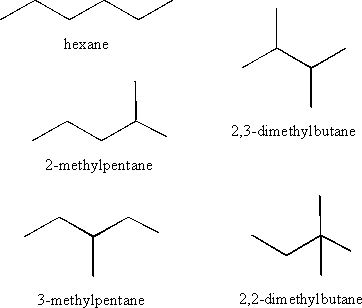
a) Write the structures of the five isomers of C6H14 and name each one.
An isomer refers to molecules that have the same chemical formula, but a different atomic arrangement.
b) Write the structure of:
i) 4 ethyl 2,2 dimethylheptane
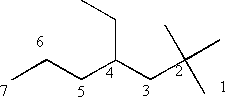
Number the molecule to give the lowest combination of substituent numbers
ii) 3 methylcyclopentene
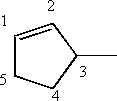
Begin numbering at the double bond and number to give the methyl group the lowest number possible
iii) trans 1 phenylpropene
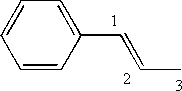
- Phenyl refers to the benzene ring as a substituent on the propene chain
- Trans indicates that the highest priority substituents on the double bond are on opposite sides (as opposed to cis, which indicates that the substituents are on the same side)
iv) 2 chloro-3-pentanol
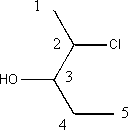
The hydroxyl group is indicated with the suffix “–ol” and the molecule is numbered to give the lowest combination
v) 2-methylhexanoic acid
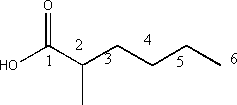
- The above molecule is a carboxylic acid, characterized by the COOH substituent and indicated by the suffix “-oic acid”
- The molecule is numbered so that the carbonyl carbon (the carbon double bonded to oxygen) is 1
vi) acetone
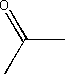
- Acetone is the common name for propanone
- The carbonyl functional group is identified with the suffix “-one”. The numbered placement of the carbonyl is not necessary, but for larger molecules, numbering is done to give the carbonyl the lowest possible number
vii) toluene
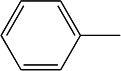
Also known as methyl benzene
viii) phenol
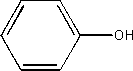
Also known as benzenol or hydroxy benzene
c) give an example of:
i) an aldehyde
Aldehydes are characterized by the functional group
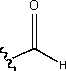
and molecules are named with the suffix “-al”
Example:
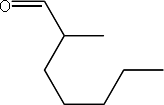
2-methylheptanal
ii) a primary amine
Amines are characterized by the NR2 functional group where R represents a hydrogen or carbon substituent. Naming is according to the substituents attached to the nitrogen followed by the “amine” suffix. N is used to denote the substituents bonded to nitrogen.
Example:
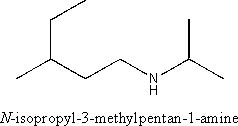
iii) an ether
Ethers are characterized by the functional group ![]() Naming is according to the “alkoxyalkane” formula where the smaller substituent is followed by “oxy” (example “methoxy”) and the larger (or more complex) substituent is named as an alkane
Naming is according to the “alkoxyalkane” formula where the smaller substituent is followed by “oxy” (example “methoxy”) and the larger (or more complex) substituent is named as an alkane
Example
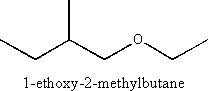
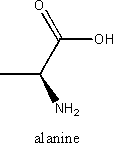
iv) an aminoacid
An amino acid is a molecule that contains both amine (NH2) and carboxyl (COOH) functional groups. There are 20 standard amino acids and the general formula for amino acids is
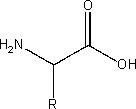
Where R represents a side chain that is specific to each amino acid.
Example:
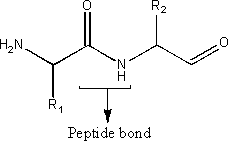
v) a dipeptide
A dipeptide is a molecule consisting of two amino acids joined by a single peptide bond. The general formula for a dipeptide is:
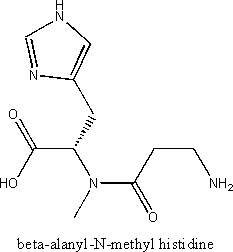
Example
d) Give an example of optical isomers.
Optical isomers are molecules that are mirror images of each other that are not super-imposable on one another. Optical isomers can occur when there is an asymmetric (chiral) carbon atom. A chiral carbon atom is one that is bonded to four different groups. A common analogy used to describe optical isomers is the example of the left and right hand, which are mirror images, but not super-imposable.
Example: S-Lactic acid and R-Lactic acid
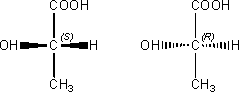
e) Give examples of the following reactions:
i) chlorination of an alkane
Chlorination of an alkane is by way of a free radical mechanism in which Cl2 is split (usually by the application of heat or UV light) into two radicals, which then replace a hydrogen on the carbon backbone. The most common product is usually an alkane with a chlorine bonded to the most substituted carbon
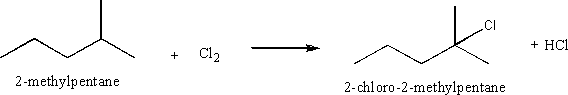
ii) the addition of iodine to an alkene
Addition of iodine (or any halogen) occurs across the double bond and adds two iodine atoms
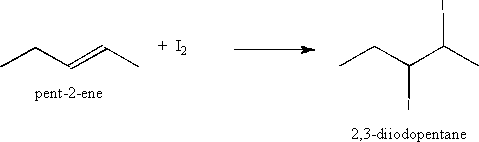
iii) an aromatic substitution reaction (e.g. the nitration of benzene)
Because of its high stability benzene does not undergo addition reactions. They undergo substitution reactions where a hydrogen atom is replaced with a functional group (e.g. halogen, SO2H, NO2).
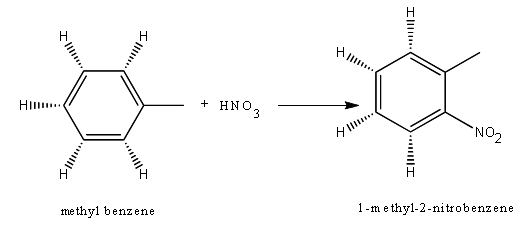
While it is possible for the nitrate group to attach at any position on the benzene ring, the reactivity and placement of the ring is affected by pre-existing substituents (in this case a methyl group). For the above example, the most common position for the nitrate to be bound is at the ortho (2nd carbon from the methyl as shown) and the para (4th carbon) positions.
iv) the formation of an ester by a condensation reaction
Esters can be formed through the reaction of a carboxylic acid and an alcohol in a condensation reaction that also produces a molecule of water (hence the name condensation).
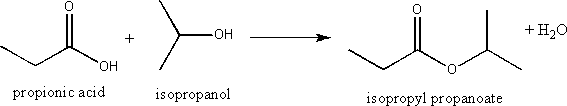
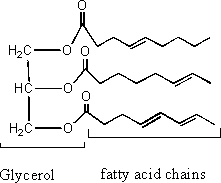
f) Explain the difference in structure between a mineral oil and a vegetable oil.
Mineral oil is primarily composed of straight chain alkanes of typically 12-40 carbons while vegetable oil is composed of triglycerides (a glycerol molecule and 3 fatty acid chains).
Mineral oil:
![]()
Vegetable Oil: