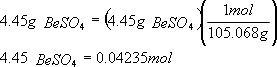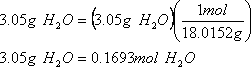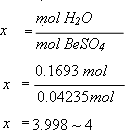Chemistry
Exercise 1
Choose the best answer.
1. Use chemical formulae in stoichiometric calculations
a) Copper consists of two isotopes: 63Cu (atomic mass = 62.930u) and 65Cu (atomic mass = 64.928u). The atomic weight of copper is 63.546. What is the percent abundance of each of the two isotopes?
Since you know that the total abundance of the two isotopes together has to
equal 1.00, you can choose the abundance of one of them to be x. In this
case, let x = the abundance of 63Cu. Therefore the abundance of 65Cu is 1-x.
Likewise, the abundance of each isotope multiplied by its molecular weight is
equal to its contribution to the atomic weight of copper.
(% 63Cu)(mol. Wt 63Cu) + (% 65Cu)(mol. Wt 65Cu) = mol. Wt. Cu.
(x)(62.930) + (1-x)(64.928)= 63.546
62.930x + 64.928 – 64.928x = 63.546
-1.998x +64.928 = 63.546
-1.998x = -1.382
x = 0.6917 ( or 69.17 %) 63Cu.
Abundance of 65Cu = 1-x
Abundance of 65Cu = 1-0.6917
Abundance of 65Cu = 0.3083 (or 30.83%)
b) When 7.50g of the hydrate BeSO4 . xH2O was heated in a vacuum, the water was driven off and 4.45g of anhydrous BeSO4 remained. What is the value of x in the formula BeSO4 . xH2O?
To answer this problem you need to know several things – how many moles of anhydrous BeSO4 do you have at the end? How many grams of water are driven off? How many moles of water does this represent? And finally, how many moles of water did you have per mole of BeSO4 at the start?
1. How many moles of anhydrous BeSO4 do you have at the end?
a) First need to know the molecular weight of BeSO4.
Mol wt. BeSO4 = (mol wt Be) + (mol wt S) + 4 (mol wt O)
= 9.012) +(32.06) + (4 x 15.994)
= 105.068
b) Now, determine the number of moles of anhydrous BeSO4
2. How many grams of water are driven off?
Grams of water driven off = wtBeSO4.H2O - anhydrous BeSO4
= 7.50g – 4.45g
= 3.05g
3. How many moles of water does this represent?
4. How many moles of water did you have per mole of BeSO4 at the start?
Therefore the value of x in the formula is 4.
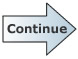
c) A sample of a compound that contains only carbon, hydrogen, and sulphur was burned in oxygen and 9.682g of CO2, 4.956g of H2O, and 3.523g of SO2 were obtained.
i. How many moles of C atoms, H atoms and S atoms did the sample contain?
Use the molecular formulas to determine the molecular weight of each product compound:
CO2 = 44.0098 g/mol
H2O = 18.0152 g/mol
SO2 = 64.0588 g/mol
Use the molecular weights to determine the number of moles of each product produced:
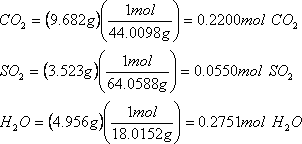
ii. What is the empirical formula for the compound?
Must determine the number of moles of each element produced:
Carbon dioxide only has one carbon, so moles carbon dioxide is equal to the moles of carbon in the original compound.
Sulphur dioxide only has one sulphur, so moles of sulphur dioxide is equal to the moles of sulphur in the original compound.
Water has two hydrogens, so the moles of water is equal to twice the number of hydrogens in the original compound (0.2751 x 2= 0.5502 moles).
Must compare number of moles to each other. The simplest way to do this is to set moles of the least abundant element as one (in this case S).
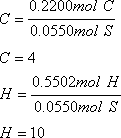
iii. What is the mass of the sample that was burned?
1. Since there is 0.0550mol of sulphur dioxide produced, and there is only one sulphur in the original compound, 0.0550 mol of the original sample must have burned.
2. Use the empirical formula to determine the molecular weight of the compound.
Mol wt sample = (4 x C) + (10 x H) + (1 x S)
= (4 x 12.011) + (10 x 1.0079) + (32.06)
= 90.183 g/mol
3. Determine the mass burned:
![]()
Thus, 4.96 g of the sample was burned.
d) Use the equation P4O10(s) + 6H2O(l) ![]() 4 H3PO4(l) to determine the number of grams of H3PO4(l) produced by the reaction of 6.00g of P4O10(s), assuming the reaction goes to completion.
4 H3PO4(l) to determine the number of grams of H3PO4(l) produced by the reaction of 6.00g of P4O10(s), assuming the reaction goes to completion.
1. How many moles are there in 6.00 g P4O10?
Molecular weight of P4O10 based on empirical formula = 283.89
![]()
Write out the formula to see how P4O10 relates to H3PO4:
P4O10(s) +6H2O(l) → 4 H3PO4(l)
So, 1 mol P4O10 → 4 mol H3PO4
And therefore 0.02113 mol P4O10 → (4)(0.02113 mol) H3PO4 = 0.08453 mol H3PO4
2. How many grams of H3PO4 are in 0.08453 mol H3PO4?
![]()
Therefore, 8.28 g of H3PO4 are produced.
e) When 0.500g of Al(s) is added to 0.100 L of 0.350 M solution of Cu(NO3)2, copper metal precipitates. The equation for the reaction is:
2Al(s) + 3Cu(NO3)2(aq) ![]() 2Al(NO3)3(aq) + 3Cu(s)
2Al(NO3)3(aq) + 3Cu(s)
i) What is the limiting reactant?
You need to determine the number of moles of each reactant that are available to react:
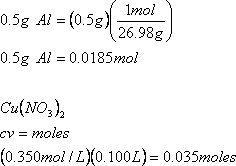
Therefore, aluminum is the limiting reactant as less moles are available to react.
ii) What mass of Cu(s) is obtained?
Assuming that the reaction goes to completion, aluminum is the limiting reactant. Therefore 0.0185 mol of aluminum are available.
The chemical reaction equation
2 Al(s) + 3Cu(NO3)2(aq) → 2Al(NO3)3(aq) + 3Cu(s)
tells you that 2 Al are needed to produce 3 Cu.
So, 1 Al → 3/2 Cu
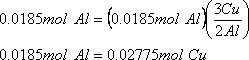
And:
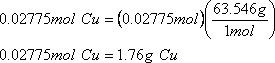
Therefore 1.76g of copper is obtained from this reaction