Chemistry
Exercise 2
2. Understand the concepts of structure, properties and periodicity, including nuclear chemistry.
a) Identify the atoms that have the following ground-state electronic configurations in their outer shell or shells:
There are a few things that you need to know to approach these questions:
1. The number of electrons in a ground state atom is the same as the number of protons (the atomic number). The atomic number determines what the element is.
2. Each electronic sublevel must be filled before going on to fill the next one.
3. The s level can hold 2 electrons, the p level can hold 6, the d level can hold 10 and the f level can hold 14.
4. The periodic table can help to show you the order in which the electrons fill their orbitals:
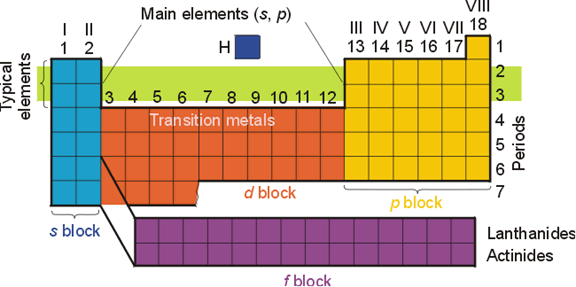
When it comes to filling orbitals and levels, you want to proceed across the periods in order, starting with the s levels of the first period. Thus, the orbitals and levels fill in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, etc.
i) 3s23p63d34s2
Work across the periods, adding electrons as you go and remembering that all the levels lower than 3s have to be full:
1s2 = 2 electrons
2s2, 2p6 = 8 electrons
3s2, 3p6= 8 electrons
4s2, 3d3 = 5 electrons
Add up the electrons (2 + 8 +8 +5 =23). The number of electrons has to be equal to the number of protons, so the atomic number of the element you are looking for is 23 (Vanadium).
ii) 4s24p3
Work across the periods, adding electrons as you go and remembering that all the levels lower than 4s have to be full:
1s2 = 2 electrons
2s2, 2p6 = 8 electrons
3s2, 3p6= 8 electrons
4s2, 3d10, 4p3 = 15 electrons
Add up the electrons (2 + 8 +8 +15 = 33). The number of electrons has to be equal to the number of protons, so the atomic number of the element you are looking for is 33 (Arsenic).
iii) 5s25p6
Work across the periods, adding electrons as you go and remembering that all the levels lower than 4s have to be full:
1s2 = 2 electrons
2s2, 2p6 = 8 electrons
3s2, 3p6= 8 electrons
4s2, 3d10, 4p6 = 18 electrons
5s2, 4d10, 5p6 = 18 electrons
Add up the electrons (2 + 8 +8 +18 + 18 = 54). The number of electrons has to be equal to the number of protons, so the atomic number of the element you are looking for is 54 (Xenon).
iv) 4s24p64d104f65s25p66s2
Work across the periods, adding electrons as you go and remembering that all the levels lower than 4s have to be full:
1s2 = 2 electrons
2s2, 2p6 = 8 electrons
3s2, 3p6= 8 electrons
4s2, 3d10, 4p6 = 18 electrons
5s2, 4d10, 5p6 = 18 electrons
6s2, 4f6 = 8 electrons
Add up the electrons (2 + 8 +8 +18 + 18 + 8 = 62). The number of electrons has to be equal to the number of protons, so the atomic number of the element you are looking for is 62 (Samarium).
4s24p65s2
Work across the periods, adding electrons as you go and remembering that all the levels lower than 4s have to be full:
1s2 = 2 electrons
2s2, 2p6 = 8 electrons
3s2, 3p6= 8 electrons
4s2, 3d10, 4p6 = 18 electrons
5s2 = 2 electrons
Add up the electrons (2 + 8 + 8 + 18 + 2 = 38). The number of electrons has to be equal to the number of protons, so the atomic number of the element you are looking for is 38 (Strontium).
b) Which member of each of the following pairs would you expect to have the higher first ionization energy?
There are a few general rules when it comes to ionization energy:
a) Ionization energy increases as you move across a period on the periodic table from left to right because the atoms are smaller due to an increase in effective nuclear charge.
b) Metals have lower ionization energies than non metals.
c) Noble gases have the ns2np6 electron configuration are very stable and have very high ionization energies.
d) Be, Mg, Zn, Cd, and Hg have full ns2 configurations, making them more stable and giving them higher ionization energies.
e) N, P and As have ns2np3 electron configurations which are also very stable and give them higher ionization energies
i) S, Ar
Ar because it is a noble gas (stable electron configuration) and is further to the right in the same period (thus smaller)
ii) Ar, Kr
Both are noble gases, but Ar is higher up (thus smaller) and will have higher ionization energy.
iii) S, As
S as it is higher up and more to the right (thus smaller).
iv) Ba, Sr
Sr is higher in the same group, therefore smaller.
v) Cs, Ba
Ba as it is further to the left in the same group (thus smaller).
vi) Sn, As
As it is further to the right (smaller), higher up the table (smaller) and has the stable ns2p3 electron configuration.
c) Give formulas for:
i) Zinc chloride.
ZnCl2 - need two chloride ions (-1) to balance the zinc (+2).
ii) Calcium chlorate.
Ca(ClO3)2 - Calcium’s +2 charge needs two chlorates to balance it (-1).
iii) Lead (II) sulfate.
PbSO4 - Lead is the +2 form (as noted by the Roman numeral II) is balanced by the -2 of the sulphate group.
iv) Potassium nitride.
K3N - The -3 of the nitride group needs three +1 potassium ions to balance it.
v) Aluminum oxide.
Al2O3 - Aluminum has a +3 charge and oxygen has a -2 charge, thus having enough Al to make +6 and enough oxygen to make -6 results in a balanced compound.
vi) Barium nitrate
Ba(NO3)2 - Barium has a +2 charge while nitrate is a -1, so two nitrates are needed to balance the barium.
vii) Iron (II) hydroxide
Fe(OH)2 - Hydroxide is a -1, so you need two to balance the +2 of the iron (indicated by the Roman numeral II)
viii) Iron (III) hydroxide
Fe(OH)3 - Hydroxide is a -1, so you need three to balance the +3 of the iron (indicated by the Roman numeral III).
ix) Aluminum sulfate
Al2(SO4)3 - Aluminum is a +3, while sulphate is a -2. Thus you need enough aluminum to total +6 and enough sulphate to total -6 to make this compound.
x) Chromium (III) nitrate
Cr(NO3)3 - Cr is a +3 (as noted by the Roman numeral III) and nitrate is a -1, so you need three nitrate to balance.
xi) Ammonium carbonate
(NH4)2CO3 - Ammonium is a +1, while carbonate is a -2. Thus you need two ammoniums to balance the charge on the carbonate.
xii) Sodium hydrogen sulfate or sodium bisulphate
NaHSO4 - The two positive ions (Na+ and H+) together make +2, while the sulphate is -2.
xiii) Sulfurous acid
H2SO3 - Two H+ are needed to balance the -2 of the SO3.
xiv) Phosphorus pentachloride
PCl5 - The 5 Cl- are needed to balance the +5 on the phosphorus.
d) Of all the elements in the third period (Na through Ar):
i) which has the largest atomic radius?
Na - Atoms become smaller as you move across the period from left to right as the effective nuclear charge (the amount of force pulling the electrons in towards the nucleus) increases with the atomic number.
ii) which has the highest first ionization energy?
Ar - as it is a Noble gas with a very stable ns2np6 electron configuration, so it is very difficult to ionize.
iii) which liberates the most energy per mole upon the addition of electrons to form the 1- anion?
Cl - because it is one electron short of the noble gas electron configuration, so it will gain the electron easily and be the most exothermic reaction.
iv) which is the most reactive metal?
Na - only one electron past the noble gas configuration – easy to lose that electron and form a stable reactive ion.
v) which is the most reactive nonmetal?
Cl - only one electron short of the noble gas configuration – eager to share electrons.
vi) which is the least reactive element?
Ar - It is a noble gas with a very stable ns2np6 configuration of electrons.
vii) how many are metals?
three - Na, Mg, and Al.