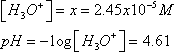Chemistry
Exercise 4
4. Use the Bronsted-Lowry theory in explaining acid-base reactions and in distinguishing between strong and weak acids and bases.
The Bronsted-Lowry theory explains acid-base reactions in terms of hydrogen ion exchange reactions: the acid is the donor of H+ions, the base is the acceptor of H+ions. As acids and bases are important entities in industrial and environmental processes, we need a good knowledge of this theory.
a) Give definitions of strong and weak acids and bases. What is the strongest acid existing in water? What is the strongest base in water? Which are the strongest acid and base existing in ethanol, CH3CH2OH?
A strong acid is one that dissociates completely in water by donating one mole of protons per mole of acid to water. A weak acid only does this to a small extent. A strong base is one that dissociates completely in water by accepting one mole of protons per mole of base to water (or by releasing one mole of hydroxide ions per mole of base). A weak base only does this to a small extent. The strongest acid in water is hydronium ion (H3O+), while the strongest base is the hydroxide ion (HO-). Any acid stronger than hydronium will dissociate and donate its protons, leaving only its weak base behind. Likewise any stronger base than hydroxide will dissociate, leaving its weak acid partner behind. The strongest acid in ethanol will be C2H5OH2+ while the ethoxide ion C2H5O- is the strongest base (the reasoning is the same as it is for water).
b) Complete the reactions by exchanging one hydrogen ion
i) H2SO4 + H2O
HSO4- + H3O+ since H2SO4is a strong acid, and so will donate a proton to water.
ii) CO32- + H2O
HCO3- + OH- since water is a stronger acid than CO32-
iii) NH2- + H2O
NH3 + OH- since water is a stronger acid
iv) HSO-4 + PO43-
HPO42- + SO42- since HSO4- is a stronger acid
c) Identify the strongest and the weakest acid in each group of three.
i) HClO4, HClO2 and HClO
strongest acid = HClO4
This can be looked up in a table of acids or bases, but can also be worked out by realizing that the number of oxygens will tend to polarize the bond attached to the hydrogen. More oxygens mean a more polar bond and one that is more likely to ionize in water. The weakest acid is HClO (for the opposite reason).
ii) H2O, H2S and HF
The strongest acid is HF (can be looked up) or worked out based on the relative ionic character of the bonds as determined by their electronegativity (as in exercise 3a). The weakest acid is H2O.
iii) H3PO4, HNO3 and HClO
the strongest acid is HNO3, while the weakest is HClO.
d) Identify the strongest and the weakest base in each group of three.
i) PO43-, HPO42- and H2PO4-
The strongest base is PO43- as looked up in an table of acids and bases or by considering which is the most likely to accept a proton from water. The proton will be strongly attracted by the greater negative charge. The weakest base is H2PO4-.
ii) -OH, -CH3 and NH2-
The strongest base will be CH3-, while the weakest base will be hydroxide (can see this on a table of conjugate bases).
iii) Cl-, Br- and F-
The strongest base will be F- since it will have the greatest tendency to accept a proton. The weakest base will be Br-. In order of strength: F- > Cl- > Br-.
e) 25.00 ml of an aqueous oxalic acid, H2C2O4 (a diprotic acid), solution of unknown concentration requires 32.40 ml of 0.250 M aqueous sodium hydroxide to just change the colour of the indicator phenolphthaleim from colourless to pink. Calculate the concentration of the oxalic acid solution.
0.162 M
As a diprotic acid, oxalic acid dissociates 2 proton equivalents per molecule. Phenolphthalein is used as an indicator to determine the equivalence point of the reaction; that is, the point at which all sodium hydroxide is reacted with oxalic acid. This means that the molar ratio of sodium hydroxide to oxalic acid will equal the molar ratio of the following balanced equation, which is 2 NaOH: 1 H2C2O4
H2C2O4 + 2 NaOH C2O42- + 2 H2O
1. Determine moles of NaOH
2. multiply by molar ratio of oxalic acid to sodium hydroxide
3. determine molarity of H2C2O4 using the volume titrated.
![]()
![]()
f) 1.00 L of a buffer solution contains 25.0 g acetic acid (CH3CO2H, Ka=1.75 X 10-5) and 25.0 g sodium acetat (NaO2CCH3). What is the pH of this buffer solution?
pH = 4.61
In order to solve this problem, you will need to consider the species that are present in solution: undissociated CH3COOH, CH3COO- (from dissociated acetic acid and sodium acetate, which dissociates completely because it is a sodium salt) and H3O+ (formed from the dissociated proton from acetic acid).
The pH of the buffer solution can be calculated using the initial concentrations of ions and the Ka of acetic acid.
- Molarity of CH3COOH (M=60g/mol) = 25 g/ 60 g/mol / 1 L = 0.42 M
- Molarity of NaO2CCH3 (M= 82 g/mol) = 0.30 M
![]()
Now we need to consider the extent to which each species listed above is found in solution at equilibrium. Keep in mind that all NaO2CCH3 dissociates completely to yield 0.3 M CH3COO- in solution. This results in the common ion effect in which the solubility of a solid is reduced if the solution already contains ions common to the solid (in this case the common ion is CH3COO-). To see this effect more clearly, it is helpful to set up an “ICE” table that lists the Initial concentrations, the net Change in concentrations and the concentrations of ions at Equilibrium. The change that occurs for each chemical species is according to stoichiometric equivalents, with reactant species showing a decrease in concentration and products increasing in concentration.
| CH3COOH | + | H20 | CH3COO- | + | H30+ | ||
Initial |
0.42M |
-- |
0.30M |
0 |
|||
Change |
-x |
-- |
+x |
+x |
|||
Equilibrium |
0.42M – x |
-- |
0.30M – x |
x |
Filling in the equilibrium values to the Ka formula gives:
![]()
Because the Ka is a relatively small value, the above equation may be simplified and the answer estimated to avoid solving for a quadratic equation. It is acceptable to simplify equations so long as the amount of ions dissociated is less than 5% of the initial molecular concentration (if you simplify, be sure to check for this at the end). The simplified equation is:
checking for the 5% dissociation rule shows that only a very small percentage dissociates;
![]()
Because the question asks for the pH, solve for the concentration of H3O+ and calculate pH